Decaffeinated Tea
Wikipedia: DecaffeinationLast Updated: Jul. 27, 2017
 Decaf Chai from Twinings, Bigelow, and Celestial Seasonings. Flavored teas like these spiced tea blends often taste better than pure decaf tea. Photo © Rusty Clark, CC BY 2.0
Decaf Chai from Twinings, Bigelow, and Celestial Seasonings. Flavored teas like these spiced tea blends often taste better than pure decaf tea. Photo © Rusty Clark, CC BY 2.0Decaffeinated tea usually suffers from inferior flavor. People seeking caffeine-free tea-like beverages but unsatisfied with the quality of decaffeinated tea might consider exploring rooibos, red raspberry leaf, or any number of other caffeine-free herbal teas. Also worth considering, decaffeinated scented and flavored teas are often more enjoyable than decaffeinated pure teas, because the tea can be blended with flavorings or other ingredients after the decaffeination process has been carried out, leaving the additional flavors intact.
Below we explore the history and safety of various decaffeination methods, focusing on ones used in tea.
Different methods of decaffeination
Decaffeination can be carried out by a number of different processes; some are safer than others, and they also have different impacts on flavor.CO2 - This method involves using carbon dioxide which has been compressed into a supercritical fluid. This method has the advantage that the only solvent used is carbon dioxide, which is completely non-toxic. CO2 is good at preserving flavor; its main downside is that it is expensive.
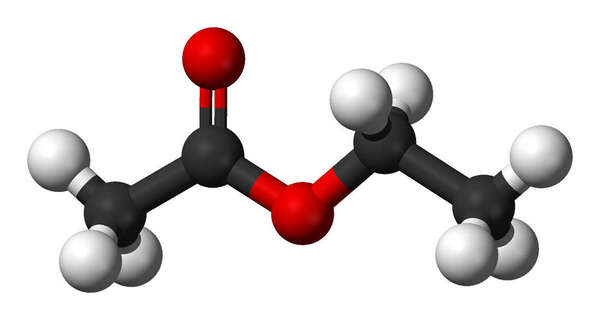
Ethyl acetate - Ethyl acetate is an organic solvent commonly used in decaffeination. It is naturally occurring, both in fruit and in tea leaves, and has a low toxicity. Ethyl acetate tends to remove more flavor from tea than other decaffeination processes; it is primarily used because of its low cost.[2]
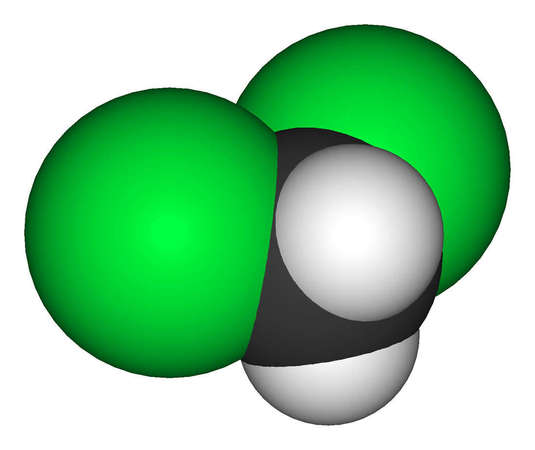
Methylene chloride - Another decaffeination process involves methylene chloride, a chlorohydrocarbon, also known as dichloromethane. The Atlantic claims that methylene chloride decaf leads to superior taste in coffee, relative to both Ethyl Acetate and CO2. But this process leaves small traces of the chemical within the decaffeinated tea leaves. Methylene chloride is toxic, and it has been shown to be carcinogenic in animals. This method is legal in the US but regulated by the FDA. Companies selling tea that has been decaffeinated by this process usually do not bring attention to it because of its bad public perception. The amount of the solvent remaining in the finished tea leaves, and the degree to which it poses a health risk, is disputed. One article advocating for its relative safety claimed that the concentration in tea is less than 1ppm [3], but an older study of decaffeinated teas found levels as high as 15.9ppm[4], beyond the US FDA's allowed threshold in food products.
Discontinued (historic) processes - Ludwig Roselius invented the first decaffeination process, which involved benzene. Benzene was later found to be toxic and carcinogenic, causing serious systemic health problems even in low quantities. Later processes involved trichloroethylene, which was discontinued after it was found to cause liver tumors in mice.[1] Fortunately for tea drinkers, the history of decaffeinated tea is relatively younger, especially in the U.S., and these unsafe processes were used primarily for coffee. Decaffeinated tea was first sold in the U.S. by Bromley Tea, which was founded in 1981, after trichloroethylene had been discontinued.
Other methods: Other less common methods of decaffeination include one using orange-peel extract.[1] There is also a process called the Swiss Water Process, for decaffeinating coffee, but this process is unique to coffee and an analagous process has not been developed for tea.
Decaf Tea Q&A
Is decaffeinated tea safe to consume?
The answer to this question depends on the decaffeination process used. CO2 and Ethyl Acetate decaffeination are completely safe. The most dangerous decaffeination methods have been long since discontinued. The only process still in use, which poses health concerns, is methylene chloride. If levels of this chemical are below regulated levels, this process is likely safe, but there have been cases of decaffeinated tea being found to contain higher levels of this compound, so if in doubt, check to see which method a company uses, and avoid tea decaffeinated by this method. Photo by Pasi Mämmelä, © Pasi Mämmelä, CC BY-SA 2.0
Photo by Pasi Mämmelä, © Pasi Mämmelä, CC BY-SA 2.0Can I decaffeinate my own tea with water?
In general, no. There are numerous blog posts claiming that most caffeine is extracted in the first 30 seconds or so of infusing a tea, and that brewing the tea briefly and discarding the water can be used to remove most of the caffeine. This technique does not work well because caffeine tends to diffuse together with flavor. For finely broken teas, this technique removes most of the caffeine but leaves little flavor. But with whole-leaf teas that retain their flavor longer, significant amounts of caffeine remain in the leaf even after longer infusions. For a safe bet, buy decaf tea, naturally low-caffeine teas, or caffeine-free herbal teas.Is decaf tea any less acidic?
Not necessarily, and this would depend on the tea and brewing strength. Tea is only mildly acidic, and there is little evidence that tea contributes to acid reflux in most people. Furthermore, there is no evidence that the caffeine in tea or coffee contributes to acid reflux. See acidity of tea and tea and acid reflux for more explanation of these topics.What is "natural decaffeination"?
A lot of tea and coffee companies market their products using terms like naturally decaffeinated. This term is not standardized or legally defined, so it doesn't say much if a company uses this term on their products. "Natural decaffeination" often refers to CO2 or water-based processes, but it can also refer to ethyl acetate or other methods.References:
1. Constantina Tzia, George Liadakis, Extraction optimization in food engineering, CRC Press, 2003.
2. Decaffeinated teas and the decaffeination process., Archive.org, from www.uptontea.com, Retrieved Mar. 4, 2016.
3. Sturdivant, Shea, Methylene chloride decaffeination: bad process: or bad press?, Tea & Coffee Trade Journal, Feb. 1, 1991.
4. Page BD, Charbonneau CF, Headspace gas chromatographic determination of methylene chloride in decaffeinated tea and coffee, with electrolytic conductivity detection., J Assoc Off Anal Chem., 1984 Jul-Aug;67(4):757-61.